2024 with 44,000 more transit truck trips through Switzerland
Mar 20, 2025 at 6:03 PM
Swisslog celebrates groundbreaking for new headquarters in Aarau
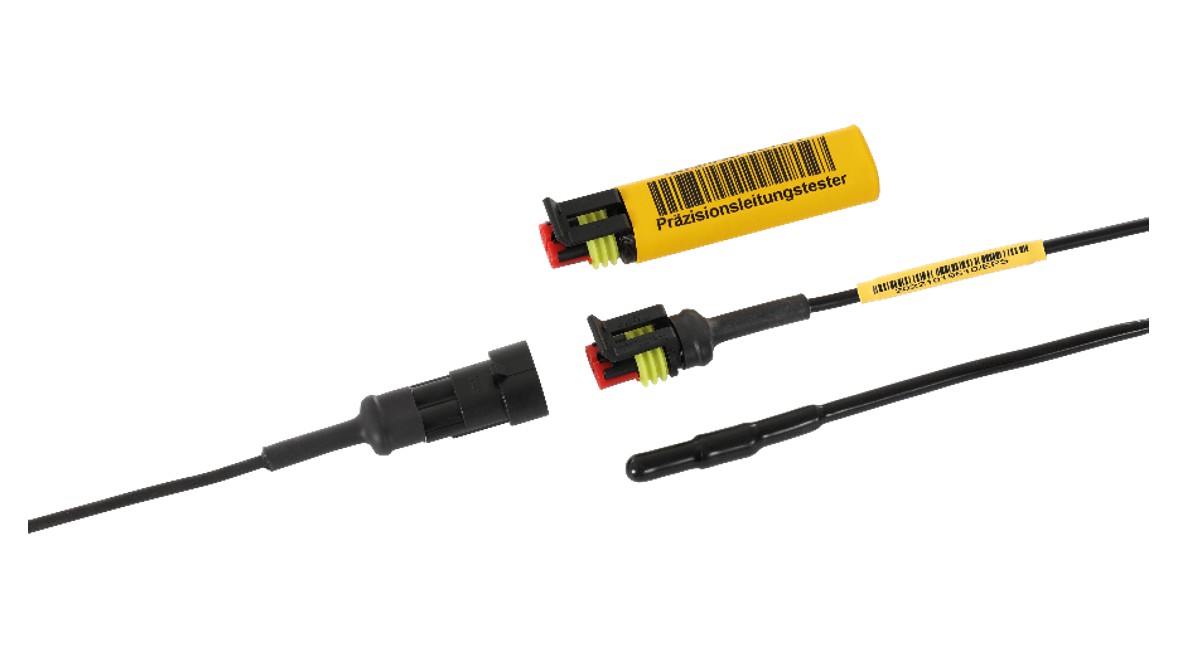
Mar 28, 2025 at 5:21 PMIn April 2024, a comprehensive validation of the calibration processes for plug sensors was conducted at Euroscan Parts & Services GmbH. The examination was carried out by the EIPL European Institute for Pharma Logistics e.K. The measure was implemented in accordance with the requirements of DIN EN ISO 13485, WHO Technical Report No. 96, 2011 Annex 9 (Chapter 4.10.1 and 6.5.2), as well as the EU GDP guidelines.
(Kiefersfelden/Mägenwil) The aim of the validation was to ensure that the calibration procedures comply with regulatory requirements and deliver highly precise, reproducible results. As part of the validation process, all relevant steps were examined – including calibration procedures, documentation, training status of the specialized personnel, and integrated quality controls. The results confirmed the consistency and reliability of the applied processes.
The successful validation underscores Euroscan Parts & Services’ ability to offer precise calibration services that meet the high demands in the medical technology, pharmaceutical, and food industries. The calibration system used is characterized by high efficiency and reliability. It enables fast and consistent calibration results while significantly reducing turnaround times compared to conventional methods.
Another advantage lies in the sustainability of the system: by avoiding unnecessary operating hours of cooling units, energy consumption is significantly reduced. This contributes to lowering operating costs while simultaneously supporting environmentally friendly processes. Additionally, downtime of vehicle fleets is eliminated, thereby increasing productivity. The system thus offers an economical and forward-looking solution for companies with high demands for precise calibration in sensitive areas.
Photo: © Euroscan